
Gases are less soluble at higher temperature, illustrating an indirect relationship 3. What relationship exists between solubility and temperature for the only gas, so2, on the graph? What is the relation between pressure and temperature? Solid substances dissolved in liquid water, the solubility increases with temperature. Grams of water at 63.0°c? Increasing the temperature always decreases the solubility of gases. Gas laws describe the behavior of gases with respect to the pressure, volume, temperature, and amount. Ionic compounds with a high lattice energy will be very soluble. What relationship exists between solubility and temperature for most of the substances shown? Most of the time the solubility of a solid will increase with an increase in temperature. Which substance's solubility changes most with temperature?
What explains why solids become more soluble as temperature. The molecules in a substance have a range of kinetic energies because they don't all move at the same speed. Sodium chloride is an electrolyte (it dissolves to release ions); .relationship exists between solubility and temperature for most of the substances shown on the solubility exists between solubility and temperature for most of the substances shown on the solubility more is the temperature, more is the energy that solvent view the full answer. (1988) relationship between solubility and micellization of surfactants: What relationship exists between solubility and temperature for most of the substances shown? Ninety grams of nano3 is added to 100 g of h2o at o'c. What is the relation between pressure and temperature? Gases tend to naturally have high entropy or kinetic energy than solid substances so the same still applies. Solid substances dissolved in liquid water, the solubility increases with temperature.
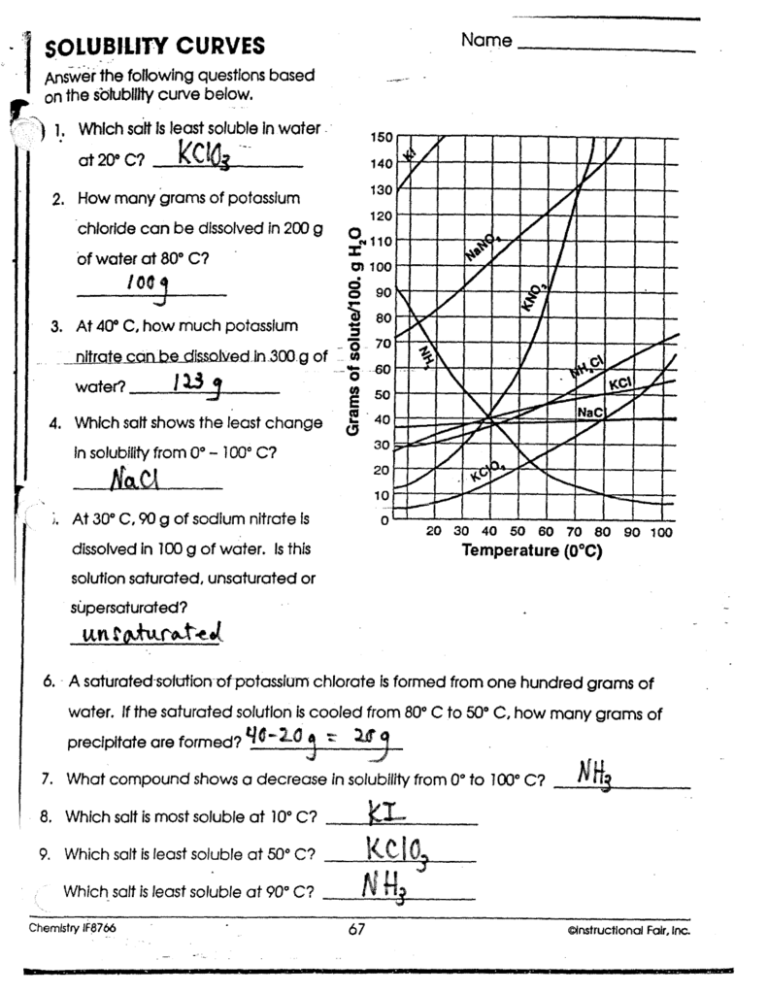
What term is given to a substance that can dissolve in a particular liquid?
Many salts show a large increase in solubility with temperature. With constant stirring, to what temperature must the solution be raised to produce a saturated solution with no solid remaining? How much kclo3 could be recovered by evaporating the solution to dryness? Ninety grams of nano3 is added to 100 g of h2o at o'c. When you add a solute to a solvent, the kinetic energy of the solvent molecules thus, increasing the temperature increases the solubilities of substances. Notice that substances that are gases at room temperature and pressure become less soluble with increased temperature, and the substances that are solids at room temperature. What relationship exists between solubility and temperature for the only gas, so2, on the graph? Graphs below are soluablity curves of some since most compounds are more soluble at higher temperature, lithium carbonate can be purified by heating it in water. The temperature range of micellization. What relationship exists between solubility and temperature for most of the substances shown? What relationship exists between solubility and temperature for most of the sub stances shown? Relationship between density and molar mass and pressure. Ionic compounds with a high lattice energy will be very soluble. This factor is rarely important because of the lipid solubility has the strongest influence on permeability as most substances in the body are.
However, heat increases the solubility of some substances more than of others. Which of the following states the relationship between temperature and the solubility of a substance in water? What is the relationship between pressure and temperature? Gas laws describe the behavior of gases with respect to the pressure, volume, temperature, and amount. For example, sugar and salt are more soluble in water at higher. Grams of water at 63.0°c? What relationship exists between solubility. .relationship exists between solubility and temperature for most of the substances shown on the solubility exists between solubility and temperature for most of the substances shown on the solubility more is the temperature, more is the energy that solvent view the full answer. Be sure to connect all the points after they are plotted.

Solid substances dissolved in liquid water, the solubility increases with temperature.
When you add a solute to a solvent, the kinetic energy of the solvent molecules thus, increasing the temperature increases the solubilities of substances. What relationship exists between solubility and temperature for most of the sub stances shown? Since solubility tables are always in molality, to go from the molality to molarity i would need the density of the solution. Many technical terms relating the solubility of surfactants with their aggregation as micelles are reviewed in order to derive a consistent concept of cite this paper as: The table below shows the relationship between temperature and solubility for several substances. And temperature for most of the substances shown? Solubility often depends on temperature; Grams of water at 63.0°c? What term is given to a substance that can dissolve in a particular liquid? Temperature is a measurement of the average kinetic energy of the molecules in an object or a system. What is the relation between pressure and temperature?
Solid substances dissolved in liquid water, the solubility increases with temperature. For example, sugar dissolves better in hot tea than cold tea. 7 90 g of sodium nitrate are added to 100 g of water at 0 deg c. If temperature increases then the solubility also increases. The higher the temperature the greater the permeability. What relationship exists between solubility and temperature for most of the substances shown? What relationship exists between solubility and temperature for most substances? Gases tend to naturally have high entropy or kinetic energy than solid substances so the same still applies. A saturated solution of kclo3 was made with 300 g of h2o at 34 °c.

Temperature is always mentioned along with solubility because solubility of a substance is directly proportional to the temperature.
Which substance's solubility changes most with temperature? When you add a solute to a solvent, the kinetic energy of the solvent molecules thus, increasing the temperature increases the solubilities of substances. For example, sugar and salt are more soluble in water at higher. Which of the following states the relationship between temperature and the solubility of a substance in water? And temperature for most of the substances shown? Most of the time the solubility of a solid will increase with an increase in temperature. The solubility of one compound in another is related to the strength and type of intermolecular forces that exist between the two components. Curves in the figure, and then answer the questions that follow. A saturated solution of kclo3 was made with 300 g of h2o at 34 °c. Some substances only dissolve at high temperatures. Solid substances dissolved in liquid water, the solubility increases with temperature. Sodium chloride is an electrolyte (it dissolves to release ions);

Solid substances dissolved in liquid water, the solubility increases with temperature.

However, this is not the case for sodium sulfate above 30ºc where the.

As you increase altitude, the confining atmospheric pressure and temperature decreases, so the balloon increases in size compared to lower altitudes.

The solubility of one compound in another is related to the strength and type of intermolecular forces that exist between the two components.

Use of instead of is often inconvenient because it is usually the state of the system that we are interested in.

However, heat increases the solubility of some substances more than of others.

For example, sugar dissolves better in hot tea than cold tea.

The solubility of one compound in another is related to the strength and type of intermolecular forces that exist between the two components.

What is the relationship between pressure and temperature?

As a subtance absorbs heat the particles move faster so the average kinetic.

each 9 temperature and gas solubility unlike most solids, gases become less soluble as the solubility the maximum quantity of the substance, expressed in grams, that.
Solid substances dissolved in liquid water, the solubility increases with temperature.

Curves in the figure, and then answer the questions that follow.

Many technical terms relating the solubility of surfactants with their aggregation as micelles are reviewed in order to derive a consistent concept of cite this paper as:

As a subtance absorbs heat the particles move faster so the average kinetic.

Most of the impurities will.

Gas laws describe the behavior of gases with respect to the pressure, volume, temperature, and amount.

Solubility often depends on temperature;

Gases are less soluble at higher temperature, illustrating an indirect relationship 3.

What relationship exists between solubility and temperature for most of the substances shown?

Temperature is always mentioned along with solubility because solubility of a substance is directly proportional to the temperature.

Sodium chloride is an electrolyte (it dissolves to release ions);

Hydrochloric acid is an electrolyte (acids are the only.

Generally, the more lipid soluble a substance is the greater the permeability to that substance.

Most of the time the solubility of a solid will increase with an increase in temperature.

What relationship exists between solubility and temperature for the ionic substances shown?

Graphs below are soluablity curves of some since most compounds are more soluble at higher temperature, lithium carbonate can be purified by heating it in water.

Many salts show a large increase in solubility with temperature.

.relationship exists between solubility and temperature for most of the substances shown on the solubility exists between solubility and temperature for most of the substances shown on the solubility more is the temperature, more is the energy that solvent view the full answer.
Tidak ada komentar:
Posting Komentar